134 23.1. Innate Immune Response
Learning Objectives
By the end of this section, you will be able to:
- Describe physical and chemical immune barriers
- Explain immediate and induced innate immune responses
- Discuss natural killer cells
- Describe major histocompatibility class I molecules
- Summarize how the proteins in a complement system function to destroy extracellular pathogens
Physical and Chemical Barriers
Before any immune factors are triggered, the skin functions as a continuous, impassable barrier to potentially infectious pathogens. Pathogens are killed or inactivated on the skin by desiccation (drying out) and by the skin’s acidity. In addition, beneficial microorganisms that coexist on the skin compete with invading pathogens, preventing infection. Regions of the body that are not protected by skin (such as the eyes and mucus membranes) have alternative methods of defense, such as tears and mucus secretions that trap and rinse away pathogens, and cilia in the nasal passages and respiratory tract that push the mucus with the pathogens out of the body. Throughout the body are other defenses, such as the low pH of the stomach (which inhibits the growth of pathogens), blood proteins that bind and disrupt bacterial cell membranes, and the process of urination (which flushes pathogens from the urinary tract).
Despite these barriers, pathogens may enter the body through skin abrasions or punctures, or by collecting on mucosal surfaces in large numbers that overcome the mucus or cilia. Some pathogens have evolved specific mechanisms that allow them to overcome physical and chemical barriers. When pathogens do enter the body, the innate immune system responds with inflammation, pathogen engulfment, and secretion of immune factors and proteins.
Pathogen Recognition
An infection may be intracellular or extracellular, depending on the pathogen. All viruses infect cells and replicate within those cells (intracellularly), whereas bacteria and other parasites may replicate intracellularly or extracellularly, depending on the species. The innate immune system must respond accordingly: by identifying the extracellular pathogen and/or by identifying host cells that have already been infected. When a pathogen enters the body, cells in the blood and lymph detect the specific pathogen-associated molecular patterns (PAMPs)
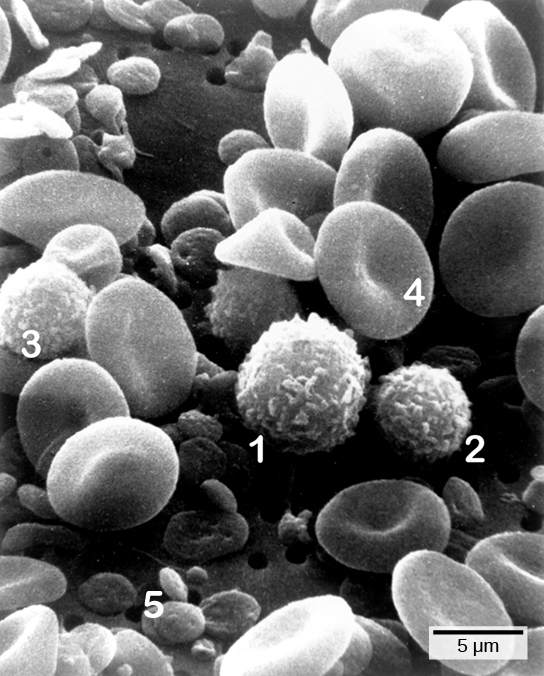
on the pathogen’s surface. PAMPs are carbohydrate, polypeptide, and nucleic acid “signatures” that are expressed by viruses, bacteria, and parasites but which differ from molecules on host cells. The immune system has specific cells, described in Figure 23.2 and shown in Figure 23.3, with receptors that recognize these PAMPs. A macrophage is a large phagocytic cell that engulfs foreign particles and pathogens. Macrophages recognize PAMPs via complementary pattern recognition receptors (PRRs). PRRs are molecules on macrophages and dendritic cells which are in contact with the external environment. A monocyte is a type of white blood cell that circulates in the blood and lymph and differentiates into macrophages after it moves into infected tissue. Dendritic cells bind molecular signatures of pathogens and promote pathogen engulfment and destruction. Toll-like receptors (TLRs) are a type of PRR that recognizes molecules that are shared by pathogens but distinguishable from host molecules). TLRs are present in invertebrates as well as vertebrates, and appear to be one of the most ancient components of the immune system. TLRs have also been identified in the mammalian nervous system.
Cytokine Release Affect
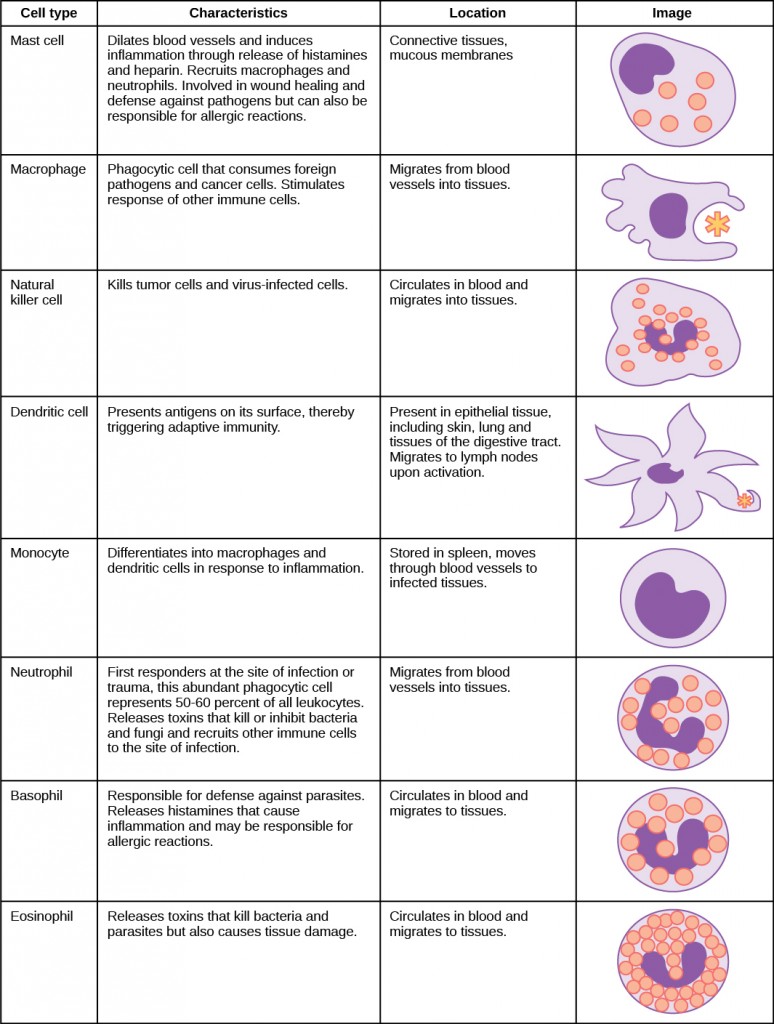
The binding of PRRs with PAMPs triggers the release of cytokines, which signal that a pathogen is present and needs to be destroyed along with any infected cells. A cytokine is a chemical messenger that regulates cell differentiation (form and function), proliferation (production), and gene expression to affect immune responses. At least 40 types of cytokines exist in humans that differ in terms of the cell type that produces them, the cell type that responds to them, and the changes they produce. One type cytokine, interferon, is illustrated in Figure 23.4.
One subclass of cytokines is the interleukin (IL), so named because they mediate interactions between leukocytes (white blood cells). Interleukins are involved in bridging the innate and adaptive immune responses. In addition to being released from cells after PAMP recognition, cytokines are released by the infected cells which bind to nearby uninfected cells and induce those cells to release cytokines, which results in a cytokine burst.
A second class of early-acting cytokines is interferons, which are released by infected cells as a warning to nearby uninfected cells. One of the functions of an interferon is to inhibit viral replication. They also have other important functions, such as tumor surveillance. Interferons work by signaling neighboring uninfected cells to destroy RNA and reduce protein synthesis, signaling neighboring infected cells to undergo apoptosis (programmed cell death), and activating immune cells.
In response to interferons, uninfected cells alter their gene expression, which increases the cells’ resistance to infection. One effect of interferon-induced gene expression is a sharply reduced cellular protein synthesis. Virally infected cells produce more viruses by synthesizing large quantities of viral proteins. Thus, by reducing protein synthesis, a cell becomes resistant to viral infection.
Phagocytosis and Inflammation
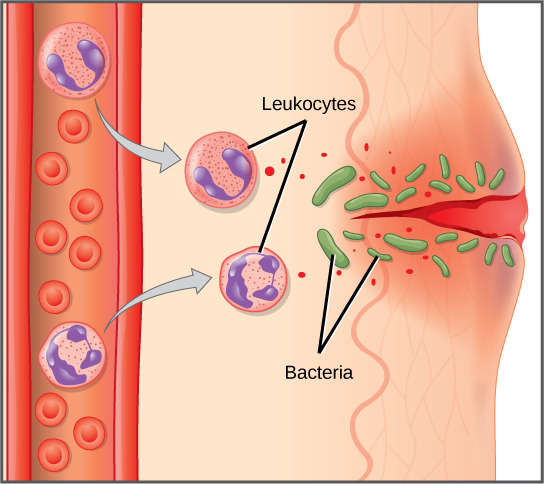
The first cytokines to be produced are pro-inflammatory; that is, they encourage inflammation, the localized redness, swelling, heat, and pain that result from the movement of leukocytes and fluid through increasingly permeable capillaries to a site of infection. The population of leukocytes that arrives at an infection site depends on the nature of the infecting pathogen. Both macrophages and dendritic cells engulf pathogens and cellular debris through phagocytosis. A neutrophil is also a phagocytic leukocyte that engulfs and digests pathogens. Neutrophils, shown in Figure 23.3, are the most abundant leukocytes of the immune system. Neutrophils have a nucleus with two to five lobes, and they contain organelles, called lysosomes, that digest engulfed pathogens. An eosinophil is a leukocyte that works with other eosinophils to surround a parasite; it is involved in the allergic response and in protection against helminthes (parasitic worms).
Neutrophils and eosinophils are particularly important leukocytes that engulf large pathogens, such as bacteria and fungi. A mast cell is a leukocyte that produces inflammatory molecules, such as histamine, in response to large pathogens. A basophil is a leukocyte that, like a neutrophil, releases chemicals to stimulate the inflammatory response as illustrated in Figure 23.5. Basophils are also involved in allergy and hypersensitivity responses and induce specific types of inflammatory responses. Eosinophils and basophils produce additional inflammatory mediators to recruit more leukocytes. A hypersensitive immune response to harmless antigens, such as in pollen, often involves the release of histamine by basophils and mast cells.
Cytokines also send feedback to cells of the nervous system to bring about the overall symptoms of feeling sick, which include lethargy, muscle pain, and nausea. These effects may have evolved because the symptoms encourage the individual to rest and prevent them from spreading the infection to others. Cytokines also increase the core body temperature, causing a fever, which causes the liver to withhold iron from the blood. Without iron, certain pathogens, such as some bacteria, are unable to replicate; this is called nutritional immunity.
Concept in Action

Watch this 23-second stop-motion video showing a neutrophil that searches for and engulfs fungus spores during an elapsed time of about 79 minutes.
Natural Killer Cells
Lymphocytes are leukocytes that are histologically identifiable by their large, darkly staining nuclei; they are small cells with very little cytoplasm, as shown in Figure 23.6. Infected cells are identified and destroyed by natural killer (NK) cells, lymphocytes that can kill cells infected with viruses or tumor cells (abnormal cells that uncontrollably divide and invade other tissue). T cells and B cells of the adaptive immune system also are classified as lymphocytes. T cells are lymphocytes that mature in the thymus gland, and B cells are lymphocytes that mature in the bone marrow. NK cells identify intracellular infections, especially from viruses, by the altered expression of major histocompatibility class (MHC) I molecules on the surface of infected cells. MHC I molecules are proteins on the surfaces of all nucleated cells, thus they are scarce on red blood cells and platelets which are non-nucleated. The function of MHC I molecules is to display fragments of proteins from the infectious agents within the cell to T-cells; healthy cells will be ignored, while “non-self” or foreign proteins will be attacked by the immune system. MHC II molecules are found mainly on cells containing antigens (“non-self proteins”) and on lymphocytes. MHC II molecules interact with helper T-cells to trigger the appropriate immune response, which may include the inflammatory response.
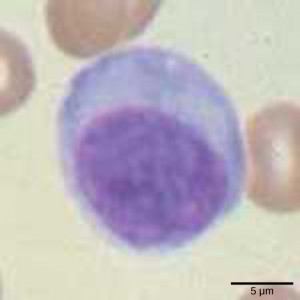
An infected cell (or a tumor cell) is usually incapable of synthesizing and displaying MHC I molecules appropriately. The metabolic resources of cells infected by some viruses produce proteins that interfere with MHC I processing and/or trafficking to the cell surface. The reduced MHC I on host cells varies from virus to virus and results from active inhibitors being produced by the viruses. This process can deplete host MHC I molecules on the cell surface, which NK cells detect as “unhealthy” or “abnormal” while searching for cellular MHC I molecules. Similarly, the dramatically altered gene expression of tumor cells leads to expression of extremely deformed or absent MHC I molecules that also signal “unhealthy” or “abnormal.”
NK cells are always active; an interaction with normal, intact MHC I molecules on a healthy cell disables the killing sequence, and the NK cell moves on. After the NK cell detects an infected or tumor cell, its cytoplasm secretes granules comprised of perforin, a destructive protein that creates a pore in the target cell. Granzymes are released along with the perforin in the immunological synapse. A granzyme is a protease that digests cellular proteins and induces the target cell to undergo programmed cell death, or apoptosis. Phagocytic cells then digest the cell debris left behind. NK cells are constantly patrolling the body and are an effective mechanism for controlling potential infections and preventing cancer progression.
Complement
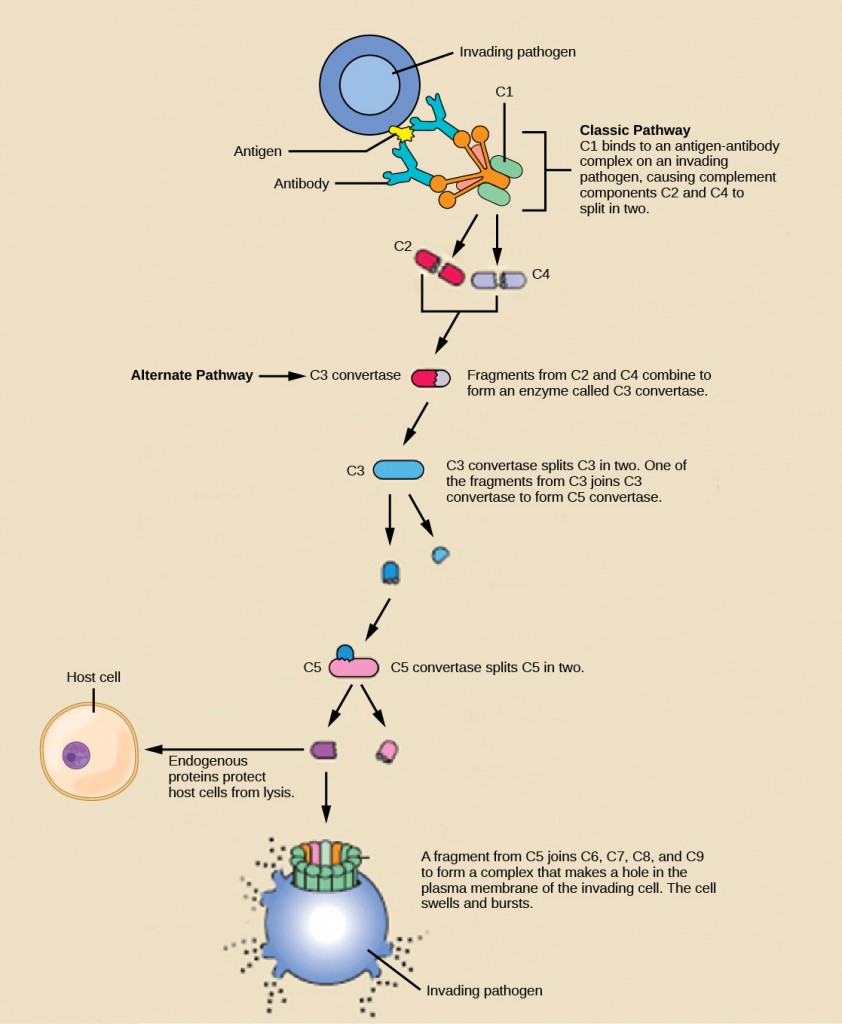
Complement proteins perform several functions. The proteins serve as a marker to indicate the presence of a pathogen to phagocytic cells, such as macrophages and B cells, and enhance engulfment; this process is called opsonization. Certain complement proteins can combine to form attack complexes that open pores in microbial cell membranes. These structures destroy pathogens by causing their contents to leak, as illustrated in Figure 23.7.
Summary
The innate immune system serves as a first responder to pathogenic threats that bypass natural physical and chemical barriers of the body. Using a combination of cellular and molecular attacks, the innate immune system identifies the nature of a pathogen and responds with inflammation, phagocytosis, cytokine release, destruction by NK cells, and/or a complement system. When innate mechanisms are insufficient to clear an infection, the adaptive immune response is informed and mobilized.
Exercises
A) high pH
B) mucus
C) tears
D) desiccation
A) bacteria
B) viruses
C) fungi
D) helminths
3. Which organelle do phagocytes use to digest engulfed particles?
A) lysosome
B) nucleus
C) endoplasmic reticulum
D) mitochondria
A) macrophages
B) neutrophils
C) NK cells
D) interferon
Glossary
- B cell
- lymphocyte that matures in the bone marrow and differentiates into antibody-secreting plasma cells
- basophil
- leukocyte that releases chemicals usually involved in the inflammatory response
- complement system
- array of approximately 20 soluble proteins of the innate immune system that enhance phagocytosis, bore holes in pathogens, and recruit lymphocytes; enhances the adaptive response when antibodies are produced
- cytokine
- chemical messenger that regulates cell differentiation, proliferation, gene expression, and cell trafficking to effect immune responses
- eosinophil
- leukocyte that responds to parasites and is involved in the allergic response
- granzyme
- protease that enters target cells through perforin and induces apoptosis in the target cells; used by NK cells and killer T cells
- inflammation
- localized redness, swelling, heat, and pain that results from the movement of leukocytes and fluid through opened capillaries to a site of infection
- innate immunity
- immunity that occurs naturally because of genetic factors or physiology, and is not induced by infection or vaccination
- interferon
- cytokine that inhibits viral replication and modulates the immune response
- lymphocyte
- leukocyte that is histologically identifiable by its large nuclei; it is a small cell with very little cytoplasm
- macrophage
- large phagocytic cell that engulfs foreign particles and pathogens
- major histocompatibility class (MHC) I/II molecule
- protein found on the surface of all nucleated cells (I) or specifically on antigen-presenting cells (II) that signals to immune cells whether the cell is healthy/normal or is infected/cancerous; it provides the appropriate template into which antigens can be loaded for recognition by lymphocytes
- mast cell
- leukocyte that produces inflammatory molecules, such as histamine, in response to large pathogens and allergens
- monocyte
- type of white blood cell that circulates in the blood and lymph and differentiates into macrophages after it moves into infected tissue
- natural killer (NK) cell
- lymphocyte that can kill cells infected with viruses or tumor cells
- neutrophil
- phagocytic leukocyte that engulfs and digests pathogens
- opsonization
- process that enhances phagocytosis using proteins to indicate the presence of a pathogen to phagocytic cells
- pathogen-associated molecular pattern (PAMP)
- carbohydrate, polypeptide, and nucleic acid “signature” that is expressed by viruses, bacteria, and parasites but differs from molecules on host cells
- pattern recognition receptor (PRR)
- molecule on macrophages and dendritic cells that binds molecular signatures of pathogens and promotes pathogen engulfment and destruction
- perforin
- destructive protein that creates a pore in the target cell; used by NK cells and killer T cells
- T cell
- lymphocyte that matures in the thymus gland; one of the main cells involved in the adaptive immune system
MHC II molecules